FDA, General Principles of Software Validation Final Guidance for Industry and FDA Staff (Release date January 11, 2002)
Author: Stan Magee CCP (Ret.)
Cover: Available
Customer Set for this product: Medical Device Firms
Format: PDF (Click here for our easy-to-modify Word® formatted version)
ISBN Numbers: 978-0-9716087-3-3 / 0-9716087-3-3
Language: English
Page count of document: 110
Provider: SEPT
Sample Pages: Available
Shipping: Available for download - Link will be provided in My ComplianceOnline section
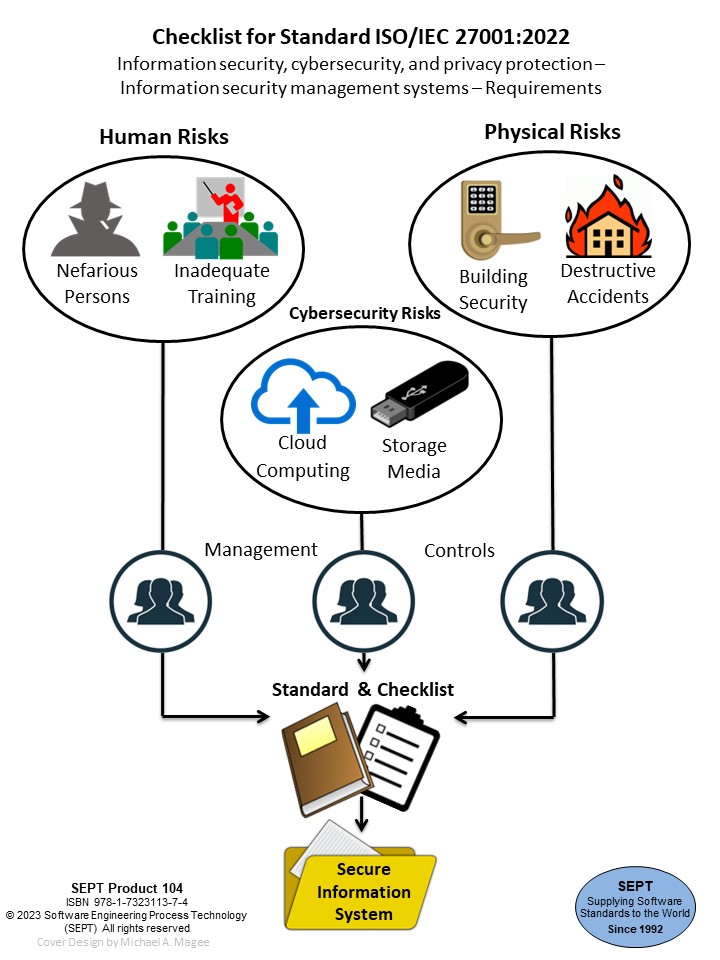
The experts at SEPT have produced a checklist for this major software engineering standard "General Principles of Software Validation" - Final Guidance for Industry and FDA staff.
The Checklist uses a classification scheme of physical evidence comprised of procedures, plans, records, documents, audits, and reviews.
The Checklist clarifies what is required for compliance by providing an easy-to-use product evidence list that will assist any software organization in meeting the requirements of this standard .
General Principles of Software Validation Checklist:
This checklist was prepared by analyzing each clause of this draft document for the key words that signify a:
- Policy
- Procedure
- Plan
- Records
- Document (Including Manuals, Reports, Scripts and Specifications)
- Audit
- Review
This checklist specifies evidence that is software or system unique.
This product supports these Software Engineering processes
- Acquisition
- Code and Test
- Configuration Management
- Documentation
- Life cycle
- Quality
- Verification and Validation
Customers of this product:
- Baxter.
- Capintec
- Carolinas Medical Center
- Cellnovo Ltd.
- CINCI. Italy
- Corning Inc
- Eradlink
- Evaheart, Japan
- Hhmpvascular.
- Logikos
- Lumina Engineering
- Medicept, Inc.
- Medtronic
- Nutramax Labs
- Power Vision Inc
- QIAGEN GmbH, Germany
- Shiseido
- Uptake Medical
Note: “International Standards (ISO) define the best of practices for Medical Device and Software firms in producing a quality product. This checklist that SEPT produces will ensure that all of the best of practices are adhered to.”
Customers Also Bought
- Evidence Product Checklist For UL 1998 Standard for Safety - Software in Programmable Components
Price: $167 BUY NOW - ISO 17025 Quality Manual Template
Price: $299 BUY NOW - Requirements for Computer System Requirements, Validation Plans, Protocols and Reports (RiskVal)
Price: $99 BUY NOW - Change Control for Validated Systems (RiskVal)
Price: $125 BUY NOW - IT Policy (RiskVal)
Price: $99 BUY NOW - Validation Plan Template for Single System Validations (RiskVal)
Price: $99 BUY NOW