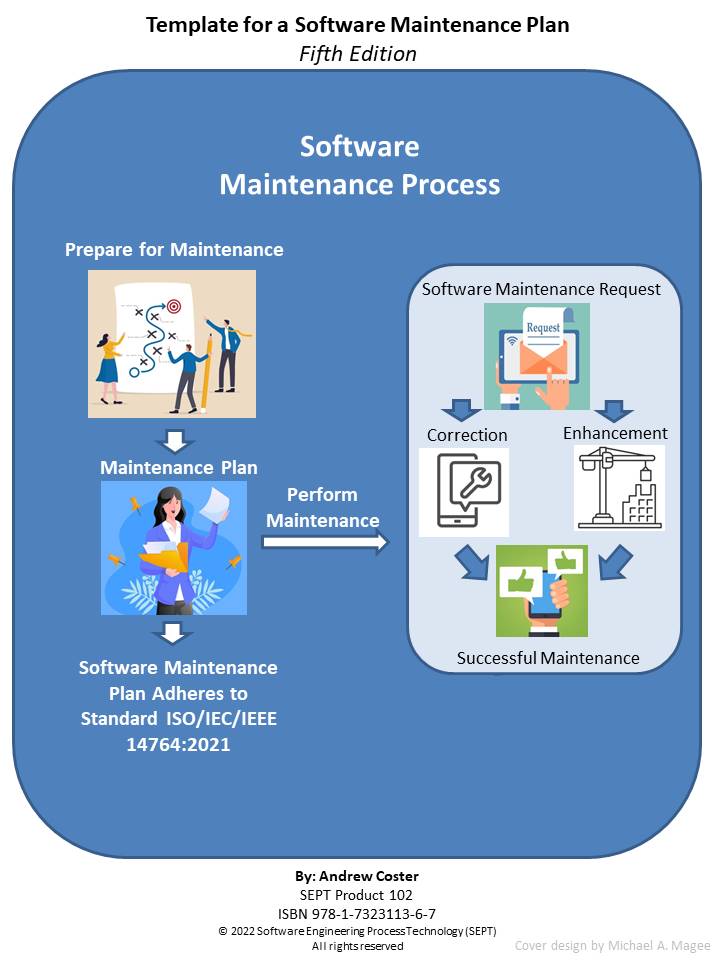
ISO 13485:2016 “Medical Devices - Quality Management Systems- Requirements for Regulatory Purposes”
Author: Andy Coster, CCP
Cover: Available
Customer Set for this product: Medical Device Firms
Format: Word® (To save money, click here for our PDF version)
ISBN Numbers: 978-0-9859732-6-1
Language: English
Page count of document: 176
Provider: SEPT
Sample Pages: Available
Shipping: Available for download - Link will be provided in My ComplianceOnline section
Overview of the base standard
ISO 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its ability to provide medical devices and related services that consistently meet customer and applicable regulatory requirements. Such organizations can be involved in one or more stages of the total product life-cycle, including design and development, production, storage and distribution, installation, or servicing of a medical device and design and development or provision of associated activities (e.g. technical support). This International Standard can also be used by suppliers or external parties that provide product including quality management system-related services to such organizations.
The updates included in the ISO 13485:2016 standard provide for a focus in utilizing risk management processes as not only part of the design and development, but also to be used throughout the quality system implementation as rationale for quality system decisions and in determining level of effort for quality system activities and deliverables.
Introduction to the SEPT checklist for implementing this standard
For 20 years (SEPT) Software Engineering Process Technology has been producing checklists for standards that address software issues. This is another checklist for a software related standard for the Medical devices industry that will aid an organization to comply with regulatory requirements.
The task of getting a new or modified medical device to market in a timely matter is a daunting task. Especially if the organization wants to apply a CE mark to its product. The last thing an organization wants in its product development cycle is to call in a Notified Body for certification and to find out that the organization is lacking the correct records or documents for the auditor to examine. SEPT experts found a 90% increase in artefacts required in the new 2016 standard compared to the previous 2003 version. This SEPT checklist list if used properly will give an organization the confidence that it has all the documentation required by this ISO 13485 standard. The checklist is a tool to ease the pain in becoming certified to ISO 13485 by clearly defining the artefacts required, whether your organization is upgrading to the new version or addressing certification to ISO 13485 for the first time.
The first step that an organization has in meeting the requirements of a quality management process standard such as Standard ISO 13485:2016is to determine what is required. Often these quality systems and technical standards are confusing and laborious because the directions contained in the standards are unclear to a lay person. In order to reduce this fog surrounding these types of standards SEPT has been producing checklists for standards since 1994. The checklists lift this fog around a standard and state what is required and suggested by the standard in a clear and concise manner. To aid in determining what is actually “required” by the document in the way of physical evidence of compliance, the experts at SEPT have produced this checklist. The SEPT checklists are constructed around a classification scheme of physical evidence comprised of policies, procedures, plans, records, documents, audits, and reviews. There must be an accompanying record of some type when an audit or review has been accomplished. This record would define the findings of the review or audit and any corrective action to be taken. For the sake of brevity this checklist does not call out a separate record for each review or audit. All procedures should be reviewed but the checklist does not call out a review for each procedure, unless the standard calls out the procedure review. In this checklist, “manuals, reports, scripts and specifications” are included in the document category. When the subject standard references another standard for physical evidence, the checklist does not call out the requirements of the referenced standard.
The checklist comes with 4 hours of free consultation, from experts that have firsthand knowledge of the underlying standard, to answer questions on the standards and checklists and is valid for 60 days after purchase of the product.
This product supports these Software Engineering processes
- Design
- Documentation
- Quality
- Life Cycle
- Safety
- Security
- Verification And Validation
Customers of this product:
- Agilent Technologies
- Applied Biosystems
- APT Services Limited
- Biofilm, Inc.
- CaP Biomaterials, LLC
- CIBA Vision
- CVAC Systems
- Diagnostic Instruments
- DNV Healthcare, Inc.
- Document Center
- Doncasters Alabama Inc
- Econmed GmbH, Germany
- ETHICON, Inc.
- Global Instrumentation Services, LLC
- Greenwaldsurgical
- Haemonetics Corporation
- HistoRx
- Homedics
- Johnson & Johnson
- Krypton Solutions
- Merit Medical Systems, Inc.
- Novartis
- Ohio Medical Corporation
- Pacific Biosciences
- Quinton Cardiology, Inc.
- Remington Medical, Inc.
- Retractable Technologies, Inc.
- Seer Pharma Pty ltd, Australia
- Siemens Medical Solutions, USA
- Spectrum Plastics Group
- Stanbio Laboratory
- Steris
- SurgRx
- Sypris T&M
- TNCO Inc.
- Vasogen Inc., Canada
- Vidar System Corporation
- ViewRay
- Waters Corporation
Note: “International Standards (ISO) define the best of practices for Medical Device and Software firms in producing a quality product. This checklist that SEPT produces will ensure that all of the best of practices are adhered to.”
Customers Also Bought
- Electronic Signatures; Final Rule-FDA 21CFR Part 11
Price: $330 BUY NOW - Guidance for Industry, FDA Reviewers and Compliance on Off-the-Shelf Software Use in Medical Devices As amended by Guidance for Industry, FDA Reviewers and Compliance on Cyber security for Networked Medical Devices Containing Off-the Shelf (OTS) Software"
Price: $167 BUY NOW - Evidence Product Checklist For UL 1998 Standard for Safety - Software in Programmable Components
Price: $330 BUY NOW - IEC 62304:2015 ''Medical Device Software - Software Life Cycle Processes''
Price: $167 BUY NOW - Checklist for FDA, Guidance for the Content of Pre-market Submissions for Software Contained in Medical Devices.
Price: $167 BUY NOW - FDA, General Principles of Software Validation Final Guidance for Industry and FDA Staff (Release date January 11, 2002)
Price: $330 BUY NOW