ISO/IEC/IEEE 90003:2018 "Software Engineering: Guidelines for the application of ISO 9001:2015 to computer software"
Author: Andy Coster, CCP (Ret.)
Cover: Available
Customer Set for this product: Firms who are applying for their 9001 certification that have software in their product
Format: PDF (Click here for our easy-to-modify Word® formatted version)
ISBN Numbers: ISBN 978-1-7323113-1-2
Language: English
Page count of document: 272
Provider: SEPT
Sample Pages: Available
Shipping: Available for download - Link will be provided in My ComplianceOnline section
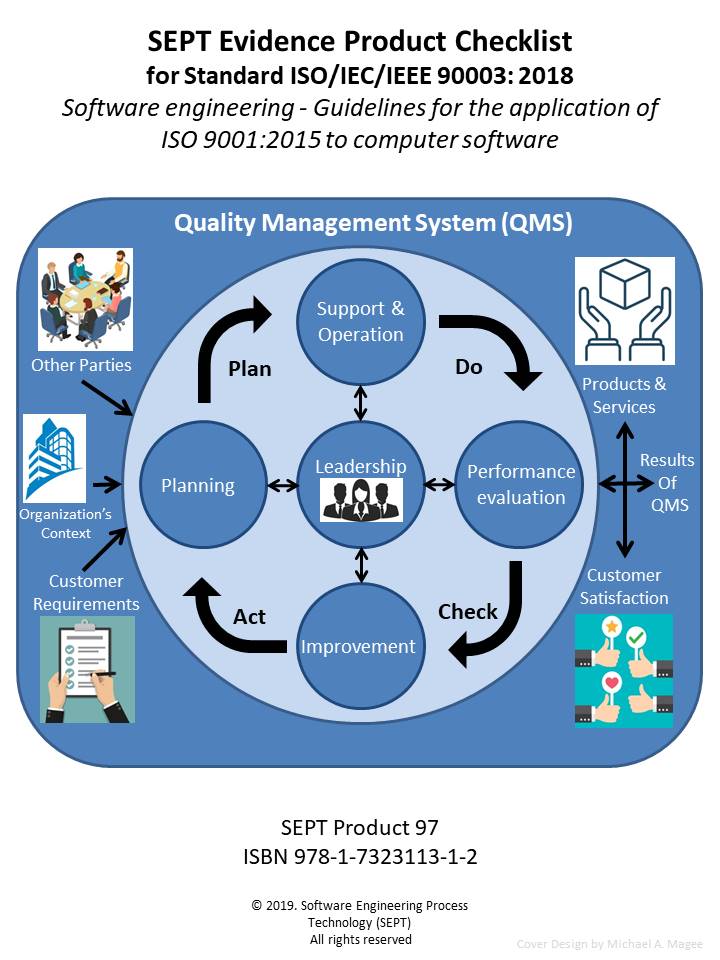
ISO/IEC/IEEE 90003 will provide your organization with guidance and support to meet the requirements of ISO 9001:2015 for systems and software developed, operated or maintained by your organization. This standard can be used by any organization, regardless of size, type and activity. To document that an organization has met the requirements of ISO 9001:2015, the standard recommends that the organization produce and use certain quality artifacts (Procedure, Policy, Plan, Records, Document, Audits and Review). However, what constitutes physical evidence (Artifacts) to meet the guidance outlined in ISO/IEC/IEEE 90003 is sometimes difficult to identify. To bridge this gap the author and SEPT experts have identified items of physical evidence called out in the standard based on their knowledge of the document and their experience in the quality field. Each item of physical evidence that was identified by these experts is listed in the checklist as; policy, procedure, plan, records, document, audits or reviews.
The SEPT checklists are constructed around a classification scheme of physical evidence comprised of policies, procedures, plans, records, documents, audits, and reviews. There must be an accompanying record of some type when an audit or review has been accomplished. This record would define the findings of the review or audit and any corrective action to be taken. For the sake of brevity this checklist does not call out a separate record for each review or audit. All procedures should be reviewed but the checklist does not call out a review for each procedure, unless the standard calls out the procedure review. In this checklist, “manuals, reports, scripts and specifications” are included in the document category. In the procedure category, guidelines are included when the subject standard references another standard for physical evidence. The checklist does not call out the requirements of the referenced standard.The author has carefully reviewed the Standard ISO/IEC/IEEE 90003:2018 and defined the physical evidence required based upon this classification scheme. SEPT’s engineering department has conducted a second review of the complete list and baseline standard to ensure that the documents’ producers did not leave out a physical piece of evidence that a “reasonable person” would expect to find. If an artifact is called out more than one time, only the first reference is stipulated. If an artifact is required by ISO 9001:2015 it appears in the checklist without appended symbol. If an item is “suggested” by ISO 9001:2015 it appears with an appended asterisk (*). If an item is “suggested” by ISO/IEC/IEEE 90003 guidelines it appears in the checklist with an appended hash (#). In this way traceability of requirements and suggested items to both standards is possible.
This product supports these Software Engineering processes
- Configuration Management
- Integration
- Life Cycle
- Maintenance And Operation
- Verification And Validation
Customers of this product:
- ASS System UK
- Baxter Healthcare
- Capintec
- Emerging, Australia
- EPS, Egypt
- Gd-Ais
- GE Rail Solutions
- Kubotek USA
- Lumina Engineering
- Meta-Fleet
- Neoprobe Corporation
- Non Profit Technology Partners
- Omniflex (UK) Ltd
- Panasonic Avionics Corp.
- Paradise-tech, Abu Dhabi
- Quality Vision International Inc.
- RTS, Inc.
- Smsocs
- Takming College
- Terumo Heart Inc.
- Therakos, Inc.
- T-Mecca Inc.
- Viasystems Group, Inc.
- Won World Pty Ltd, Australia
Note: “International Standards (ISO) define the best of practices for Medical Device and Software firms in producing a quality product. This checklist that SEPT produces will ensure that all of the best of practices are adhered to.”
Customers Also Bought
- 13485 P-841 Root Cause Analysis
Price: $39 BUY NOW - 13485 P-852 Corrective Action
Price: $39 BUY NOW - 13485 P-853 Preventive Action
Price: $39 BUY NOW - Integrating 9001:2015+14001:2015 PowerPoint
Price: $149 BUY NOW - 9001:2015/14001:2015 EMS & QMS Documentation
Price: $797 BUY NOW - ISO 14001:2015 - 9001:2015 Small Business Package
Price: $997 BUY NOW