- By: Staff Editor
- Date: July 31, 2018
Access Regulatory Compliance Training sessions led by expert panelists below.
Compliance Webinars |
Virtual Seminars for Professionals
Cleanroom Reopening after a Worst-case event - Best Practices
Some cleanrooms are reopened after a shutdown. Reasons for the shutdown could be worst case events such as bad weather conditions, power outage, maintenance schedule or human errors. When the cleanroom is reopened, it is essential to verify that the room meets the expected standards. Failure to restore the cleanroom to acceptable conditions may result in rejections during inspections.
Before you release a cleanroom for manufacturing ensure that it meets requirements as prescribed in various standard namely.
- The room should meet all the ISO-14644 (including ISO-14644 part 3) specs for particles and air control.
- Airflow Test
- Air Pressure Differential Test
- Temperature Test
- Humidity Test
- Recovery Test
- Contamination Leak Test
- HEPA Filters (Annual Testing)
- EU Monitoring Guidance
Cleanroom safety and control knowledge is very much required for any biopharma manufacturing facility as it can attract many warning letters if environmental control is not up to mark.

Cleanroom Best Practices
- Identifying common sources of bioburden and controlling them
- Understanding and implementing cleaning and disinfection methods
- Bringing the cleanroom online
Understanding bioburden, the common sources and controlling them
- Bioburden is a term used to describe the microbial numbers on a surface (or complete item) or inside a device or from a portion of liquid – Pharmaceutical biology, 2016. To maintain the bioburden levels in clean rooms, it is critical to control all the sources of contamination. Sources include people, product, packaging and cleaning materials.

Figure 1Types of Contamination
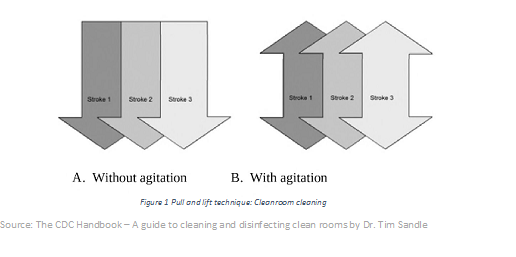
Understanding and implementing cleaning and disinfection methods in proper frequency
- Cleaning of surfaces by applying neutral detergents with gentle agitations.
- Disinfection by applying a suitable biocide by either mopping spraying or wiping.
- Biocide or detergent solutions must be prepared according to validated SOPs.
- Application of detergents or biocide agent should be to the whole of the surface and avoid spreading of any contamination present
- Cleaning and its frequency should be as per the cleaning order documented in SOPs.
- Staff training is critical for successful implementation
Bringing the cleanroom online
- Cleaning debris, dirt, soiling, particles
- Single or Double Cleaning with a formulated cleaner
- Acidic, Neutral, or Basic Cleaner can be utilized
- A Neutral cleaner is often used to prevent any substrate compatibility issues
- Goal: Clean Pristine Surfaces
For a detailed live online training of cleanroom regulations, best practices, and case studies, you may wish to sign up for the webinar ’Gaining and Re-Establishing Control of Your Cleanroom’. Mr. Polarine is a technical service manager at STERIS Corporation. He has been with STERIS Corporation for seventeen years.
You may also wish to browse through our list of webinars designed for life sciences personnel
.





















